Action for Primates

|
Action for Primates |

|
|
The following are take action items we have posted in 2024. See elsewhere for take action alerts from other years. In addition to the Take Action entries below, you can click here for petitions you can sign and share to help non-human primates around the world.
Index of action alerts; select date & title to access:
23 December 2024: Cambodian long-tailed macaques killed in alcohol research in USA

Twenty-three young male long-tailed macaques were purposefully addicted to alcohol and killed in an inhumane experiment at the Oregon National Primate Research Center (ONPRC) in the USA (Kuah et al 2024). The recently published work was approved by the ONPRC animal use committee. It was publicly funded by the National Institutes of Health (grants AA026289, AA013510, AA010760, AA019431 and OD011092, totalling several tens of millions of US dollars), as well as the National Institute of Food and Agriculture and Oregon Agricultural Experiment Station.
It is already known that alcohol (ethanol) is responsible for reducing bone quality in those humans who drink alcohol in substantial amounts. Rather than continuing to study this population of humans, these researchers used these twenty-three young long-tailed macaques, who originated in Cambodia, as surrogates for humans and forced them to become 'alcoholics'. They did this by increasing the concentration of ethanol in the only source of water available to the macaques, until the macaques were 'voluntarily' drinking water containing a substantial concentration of the drug after about four months. These now addicted macaques were then allowed to drink alcohol-laced (4% weight/volume) water for 22 hours of every day for over six months (although some were non-drinking 'controls'). Every five days, blood was taken from a leg vein. Added to this abuse, the macaques were individually housed in small, stainless steel cages with no meaningful social contact with others, especially no ability to touch or groom or otherwise physically interact with others, something that is not only incredibly inhumane, it is critical to the welfare and well-being of this species.
At the end of the 'experiment', all the macaques were killed (euphemistically referred to as euthanized
) in order to get their leg bones. There was no mention of the impact six months of heavy alcohol consumption had on the welfare or well-being of the macaques.
We are appalled by not only the continued lack of humanity and compassion demonstrated by these researchers, who have been doing alcohol research for many years using non-human primates, but also by the lack of scientific credibility of the experiments concocted by them. The researchers are not alone in this given that it is the funding agencies that continue to support such immoral work.
This experiment is deeply flawed, not only morally, but also because the macaques were not even allowed any meaningful physical exercise, something that would substantially impact bone quality. Further, this precise intake of ethanol by macaques under completely artificial captive conditions is so different from the situation for human alcohol consumers as to be ludicrous. The researchers concluded that their results suggest that by suppressing repair of fatigue microdamage to bone matrix or other outcomes linked to mean tissue age, decades of heavy alcohol consumption, common in humans, may lower bone quality.
The acknowledgement that heavy alcohol consumption is common in humans begs the question of why that population is not studied instead of concocting inhumane and costly (macaque lives and financial and other resources) experiments.
The public are repeatedly told that non-human primates are used in research only when absolutely necessary and only when there are no other alternatives available. This shameful experiment, which resulted in substantial suffering for the monkeys, followed by being killed, demonstrates the meaningless nature of such assurances and a lack of commitment to stop using non-human primates, something clearly contrary to the Replacement criterion for the 3Rs professed to be enforced by animal use committees dealing with federally funded research projects. Other researchers in the field of substance abuse have concluded that non-human primates cannot be used to 'model' the human situation (Magliaro & Ahluwalia 2022).
What you can do to help:
Cited information:
...the impact of social, psychological and economic factors that determine the onset and persistence of addiction (and recovery from it) in humans. Indeed, the predictive validity of animal models of addiction is poor, with few effective treatments deriving from animal studies. ...the principal argument against animal experiments in the field of substances of abuse is that addiction in humans is a complex disorder which encompasses psychological, social and emotional variables that are impossible to model in animals.
Information on NIH grant support (funding) is taken verbatim from relevant publications. If you have difficulty with any links provided, you can do your own search through the NIH RePORTER site: https://reporter.nih.gov/, by copying and pasting the grant number into the Search field on the form.
Be aware that some grants include funds for more than experiments on non-human primates.
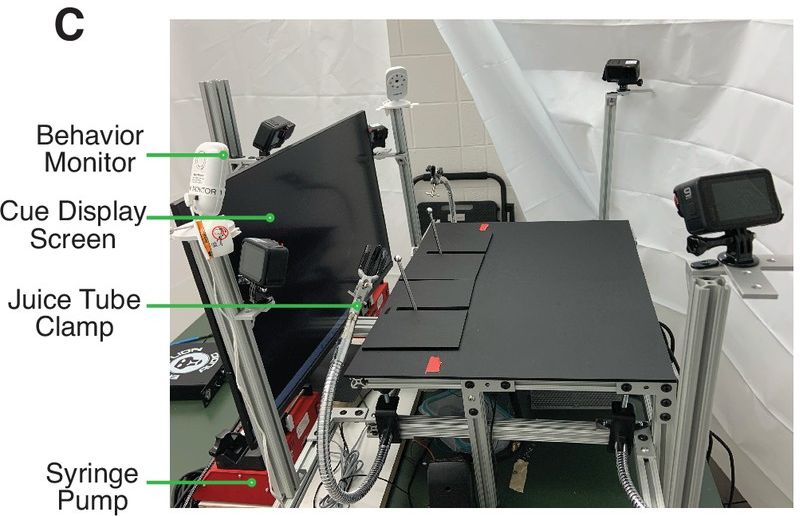
26 November 2024: Highly invasive brain surgery done to produce apparatus to study marmoset behaviour

Researchers at Yale University in the USA have developed a new 'tool' for studying marmoset behaviour (Meisner et al 2024). This research involved highly invasive brain surgery and water deprivation, all approved by the Yale Institutional Animal Care and Use Committee (Yale University IACUC Protocol #2023-20163). Multiple agencies funded the work including National Science Foundation (DGE2139841), Simons Foundation Autism Research Initiative (SFARI 875855), Wu Tsai Institute at Yale University, National Eye Institute (EY026878) and National Institute of Mental Health (MH126072, the latter two totalling millions of US tax payer dollars.
Using the marmoset as a 'model' for human behaviour, the impetus for this study was to develop a methodology that would fully capture the nuances of marmoset social interactions and cooperative behaviors
. An apparatus called MarmoAAP
was developed. It allows two marmosets in adjacent, transparent enclosures to observe each other and coordinate their actions so they can simultaneously pull levers and both receive a reward
. Seven adult common marmosets (four females, three males) were trained
to perform lever-pulling tasks. In order for the marmosets to 'cooperate', they were deprived completely from access to water. Further, their morning food was withheld for up to three hours. If they pulled the levers within certain intervals, they were given a juice reward.
One of the marmosets was subjected to two episodes of severely invasive head surgery. The researchers stated this in a rather casual manner, as if it was an innocuous 'gift': One animal received a head chamber implant and craniotomy
. During the first surgery, a head chamber
was implanted into the skull. After two weeks, a craniotomy was carried out in order to implant an electrode device onto the skull. This device was covered with a cap (see figure 1a reproduced in part above) and then used during testing to drive probes (electrodes) into the brain for recording purposes.
During testing sessions, the marmosets were forced to sit in a restraint device. The ultimate fate of the marmosets, including the individual who was surgically mutilated, was not stated.
The researchers claimed that their newly developed apparatus (see figure 1c reproduced in part here) bridges the gap between traditional animal behavior methodologies and the demand for increased precision and adaptability in behavioral research
. This was to facilitate burgeoning efforts to engineer genetically modified marmosets
and would contribute in a major way to study of marmoset social behaviors in the field
. The intent, of course, is to continue using the marmoset as a surrogate for studying human behaviour.
The public are repeatedly told that non-human primates are used in research only when absolutely necessary and only when there are no other alternatives available. This project demonstrates with clarity that such claims are simply not true. Not only is the marmoset substantially different from humans in terms of brain anatomy and function, there is no reason why humane, morally defensible studies cannot be done directly on humans themselves. That is the only way to learn and understand human behaviour, particularly because there can be conversations between the researchers and the subjects, something not feasible with marmosets.
The marmosets in these studies had to endure the stress of captivity, one with the added brutality of several episodes of invasive head surgery, and restraint for what can only be considered frivolous reasons and clearly contrary to the Replacement criterion for the 3Rs.
The information in non-human primates is irrelevant to people given the fundamental differences between the two species. Other researchers have been critical of the use of non-human primates to 'model' the human (Lahvis 2023; Petticrew & Smith 2012; Sapolsky 1993). Primatologists have also spoken out against invasive research using non-human primates (Padrell et al 2021).
What you can do to help:
Cited information:
We need healthy controls, not psychologically broken ones, to benchmark our disease models. And we need the animals used as disease models to be otherwise healthy because we lack the scientific capacity to separate the biology of a nuanced disorder, like autism or ADHD, from confounding factors like the mental damage caused by incarceration...researchers lured us into believing the ridiculous — that brain development behind steel bars is not only normal but natural enough to be relevant to evolutionary changes occurring outside the lab. Yet their monkeys experienced no full spectrum of color, no natural movement like the rustling of leaves, and no passing landscape...[normalizing] the idea that monkeys naturally live inside telephone booths, not in the vast, dynamic, and aesthetically complex expanses of nature. What bothers me most is that the scientific community expresses so little concern about whether we're chasing artifacts of confinement.
Here, we will review previous studies showing that primates have complex behaviour and cognition, and that they suffer long-term consequences after being used in invasive research.
Although some invasive studies have allowed answering research questions that we could not have addressed with other methods (or at least not as quickly), the use of primates in invasive research also raises ethical concerns. In this review, we will discuss (i) recent advances in the study of primates that show evidence of complex behaviour and cognition, (ii) welfare issues that might arise when using primates in invasive research, (iii) the main ethical issues that have been raised about invasive research on primates, (iv) the legal protection that primates are granted in several countries, with a special focus on the principle of the 3Rs, and (v) previous and current attempts to ban the use of primates in invasive research. Based on this analysis, we suggest that the importance of a research question cannot justify the costs of invasive research on primates, and that non-invasive methods should be considered the only possible approach in the study of primates. [emphasis added]
Primatologists themselves have warned repeatedly about over-generalising from primate data to human societies...the data may not even be generalisable between similar species of monkey, as comparative research and field studies suggest that there are striking differences in group composition, social spacing, dominance and aggression between species...The social and hierarchical behaviour of Macaca fascicularis, the species used in many of these studies, may not therefore even be representative of all of its own genus, which raises doubt about extrapolation to higher primates.
Please refer to the original paper for full quotes and context of this paraphrasing:it may be questioned whether animal models can ever truly mimic human psychiatric disorders...primates, with their complex social lives, cognitive skills, and emotional nuances, have been particularly attractive objects of psychiatric research interest...The problem arises from the circuitous and often tragic routes by which primates come to find themselves in laboratories...This necessitates a lengthy and traumatic transport of primates...[deaths or illness during transport] at least partially reflect the immunosuppressive effects of the stressfulness of capture and transport...survivors (and their descendents) may be the most physically and psychiatrically robust of individuals, or may, alternatively, remain permanently weakened or damaged by these vicissitudes.
Craniotomy:
This is a surgical procedure to access the brain. Typically, an area on the head is shaved. An incision is made through the skin and tissues underlying it, down to the surface of the skull, in such a way as to create a flap that can be pulled away to expose the skull. Depending on what type of access to the brain is required, holes may be drilled through the skull into the brain cavity, for example for injections, or a portion of the bone may be cut out in order to implant a bank of electrodes. When done, bone cement may be used to fill in the hole in the skull and the overlying tissues sutured.
29 October 2024: Macaques used as human surrogates to study effects of cocaine on behaviour
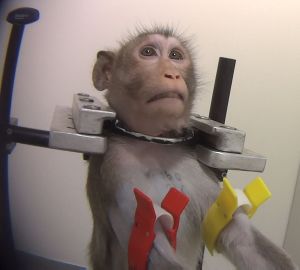
Twelve long-tailed macaques (6 females and 6 males) were used as surrogates for humans in an experiment at Wake Forest University in the USA (Allen et al 2024), to determine the effects of cocaine on behaviour. This inhumane and scientifically nonsensical work was paid for using US tax payer funds through grants DA006634, DA017763, DA053776 and DA060614 from the National Institute on Drug Abuse, a branch of the National Institutes of Health. The aggregate amount of the grants is in the many millions of US dollars. The subjugation and experimentation were approved by the Animal Care and Use Committee of Wake Forest University.
The macaques, described as cocaine-naïve
, had previously been 'trained' to respond to various tasks through the use of food rewards. For this experiment, they had catheters permanently implanted surgically under their skin in order for them to inject themselves with cocaine, euphemistically referred to by the researchers as self-administration
. In order to restrain the macaques for the testing phase of the experiment, aluminium collars were encircled around their necks and they were immobilised in a primate restraint chair
. It was not stated explicitly, but the usual reason for fitting neck collars relative to 'primate chairs' is so that a pole can be attached and the individual dragged to the chair. This pole and collar method of restraint and 'training' is considered highly inhumane even by people in the research community.
Once behavioural training was completed, the macaques were further 'trained' to 'self-administer' cocaine, which is generally done using passively coercive methods. After this, the macaques were held in the restraint device and tested in small, sound-attenuating chambers. They had to respond to a touch screen and their choices were evaluated based on how much cocaine they were 'self-administering'.
There was no demonstrated empathy for what these macaques had to endure nor any mention of their ultimate fate, or the effects of cocaine in general or when (and if) there was no further access to cocaine.
There is no shortage of humans using cocaine and there is no question that these individuals would make the 'perfect' subjects to study the behavioural effects of the drug. Despite this, the researchers concluded that these data [from the macaque experiment] provide novel insight into the relationship between cognitive abilities and cocaine self-administration and help inform research examining behavioral and/or pharmacological interventions aimed at reducing cocaine misuse
. There seemed to be no consideration for the fact that the macaques were responding mechanically, without any understanding for what they were doing. Even the journal in which the macaque study was published has stated that Authors should refrain from the use of language to imply that they are able to replicate in laboratory animals complex neuropsychiatric disorders or traits that are unique to the human species
, the underlying message being that non-human animals are not 'models' of humans. There also was no intimation by the macaques study researchers that other researchers in the field have concluded that using non-human animals such as non-human primates in substance abuse research is not only inhumane, but is also ineffective and has not resulted in the 'cures' or 'control' promised by the research industry (Magliaro & Ahluwalia 2022).
Once again, researchers are being paid to concoct useless and inhumane experiments involving non-human primates. The funding agencies are largely responsible for this. If they, instead, paid researchers to find and use innovative, humane and human-relevant methods of investigation, this would advance human health and welfare exponentially. The taxpayer needs to insist that this be the norm. Moreover, not only is the information in macaques irrelevant to people given the fundamental differences between the two species, humane and ethical clinical studies have been and can continue to be done on human patients and volunteers in order to get data that are directly applicable to people. This approach is not only humane and fiscally responsible, it is consistent with the Replacement criterion for the 3Rs, something the research community claims to follow.
What you can do to help:
Cited information:
...the impact of social, psychological and economic factors that determine the onset and persistence of addiction (and recovery from it) in humans. Indeed, the predictive validity of animal models of addiction is poor, with few effective treatments deriving from animal studies. ...the principal argument against animal experiments in the field of substances of abuse is that addiction in humans is a complex disorder which encompasses psychological, social and emotional variables that are impossible to model in animals.
Pole and collar:
This refers to placing a permanent metal collar around the neck of the individual. The collar has openings which can be 'grabbed' by the end of a specially designed pole. The individual then can be dragged around by the neck until they acquiesce to being manoeuvred in this manner. Almost all individuals vigorously object to being treated in this manner and it can take many weeks for them to acquiesce. Some become too aggressive for this method to be used.
Primate chairs:

So-called 'primate chairs', such as those manufactured by Crist Instrument Co, are restraint devices which secure the individual in an unnatural sitting position. Further restraint is often used through a neck plate which goes around the neck to prevent side-to-side or front to back movements of the head. The head may be severely restrained through the use of a post surgically implanted into the skull and held by part of the chair. Depending on the procedures being done, the arms and legs may be secured further by taping them to the chair supports.
Information on NIH grant support (funding) is taken verbatim from relevant publications. If you have difficulty with any links provided, you can do your own search through the NIH RePORTER site: https://reporter.nih.gov/, by copying and pasting the grant number into the Search field on the form.
Be aware that some grants include funds for more than experiments on non-human primates.
14 October 2024: Rhesus macaques subjected to extreme head surgery and restraint in US research

Two adult rhesus macaques were subjected to highly invasive head surgery and extremely severe restraint as surrogates for humans to study learning from symbolic outcomes
(Tang et al 2024). The experiment was publicly funded by and done at the National Institute of Mental Health (NIMH) in the USA, using grant MH002928. This grant has totalled many millions of US dollars over the last several years. Additional funding was through the Brain & Behavior Research Foundation. This barbaric treatment of the macaques was approved by the animal use committee of the NIMH.
The macaques, one female and one male, were each 8-10 years old. They were pair-housed when possible
. They were subjected to invasive head surgery during which a craniotomy was done to implant electrodes into each of their brains and a head post implanted in their skulls. After surgery, they were trained
to respond to stimuli presented on a monitor while subjected to severe body restraint – they were strapped into a primate chair
with their heads immobilised by clamping the implanted head post. They were subjected to what the researchers euphemistically referred to as water control
, which means the macaques were deprived of water or other fluids sufficiently to cause them to work for juice rewards (earned their juice by performing the task
). After 'training', they were again subjected to chair and head restraint in order to make recordings while they were tested. No mention was made of their fate after the experiment ended.
The authors cited many studies on learning in humans. None of their findings in the macaques can be uncritically translated to humans. Importantly, the macaques had no understanding of what was happening nor of what was expected of them. Instead, they simply responded mechanically in order to get a juice reward. Behavioural studies of this kind require the ability to converse with the subjects in order to learn all the information available, something not feasible in the macaques.
The public are repeatedly told that non-human primates are used in research only when absolutely necessary and only when there are no other alternatives available. This shameful experiment, which resulted in substantial suffering for the monkeys, demonstrates the meaningless nature of such assurances and a lack of commitment to stop using non-human primates. Not only is the information in macaques irrelevant to people given the fundamental differences between the two species, humane and ethical clinical studies have been and can continue to be done on human volunteers in order to get data that are directly applicable to people. The macaques in this experiment had to endure the stress of captivity, virtual social isolation, major survival surgery and severe restraint for what can only be considered frivolous reasons and clearly contrary to the Replacement criterion for the 3Rs.
What you can do to help:
Cited information:
Craniotomy:
This is a surgical procedure to access the brain. Typically, an area on the head is shaved. An incision is made through the skin and tissues underlying it, down to the surface of the skull, in such a way as to create a flap that can be pulled away to expose the skull. Depending on what type of access to the brain is required, holes may be drilled through the skull into the brain cavity, for example for injections, or a portion of the bone may be cut out in order to implant a bank of electrodes. When done, bone cement may be used to fill in the hole in the skull and the overlying tissues sutured.
Primate chairs:

So-called 'primate chairs', such as those manufactured by Crist Instrument Co, are restraint devices which secure the individual in an unnatural sitting position. Further restraint is often used through a neck plate which goes around the neck to prevent side-to-side or front to back movements of the head. The head may be severely restrained through the use of a post surgically implanted into the skull and held by part of the chair. Depending on the procedures being done, the arms and legs may be secured further by taping them to the chair supports.
Head posts:

As the name implies, these are posts that are attached to the skull. Surgery is done on the head to dissect through skin and other tissues down to the surface of the skull. The post is cemented or screwed in place. Once healing has occurred, the post is used to restrain the head while recordings or other experimentation is done. It is almost always combined with a neck plate so that the non-human primate cannot move their heads.
Information on NIH grant support (funding) is taken verbatim from relevant publications. If you have difficulty with any links provided, you can do your own search through the NIH RePORTER site: https://reporter.nih.gov/, by copying and pasting the grant number into the Search field on the form.
Be aware that some grants include funds for more than experiments on non-human primates.
24 September 2024: Baboon infants cruelly killed in human Western-style diet research in USA

Twenty-four female hamadryas baboons were used as surrogates for pregnant human females and fed different diets before and during pregnancy and their infants killed near-term (Bertossa et al 2024). The experiment was carried out at the Southwest National Primate Research Center in San Antonio, Texas and was paid for primarily by the National Institutes of Health through grants AG057758, HD021350 and RR021367, totalling several millions US dollars, and also included funding from the Australian Research Council. Although the site of the work was in the USA, many of the authors listed the University of South Australia as their affiliation. The protocols were approved by the Texas Biomedical Research Institute, University of Texas Health Science Centre at San Antonio Institutional Animal Care and Use Committee.
Twenty-four female hamadryas baboons about 11 years old were placed on a control diet (12 individuals) or a high fat-high energy diet (so-called Western-style diet) (12 individuals). They were started on these diets about nine months before mating and continued throughout pregnancy. The goal was to see the effects of the high fat-high energy diet on the hearts of the foetuses.
At about 165 days gestation, the baboons were anaesthetised, their uteruses were incised and their near-term foetuses (normal gestation for hamadryas baboons is about 170-173 days) were removed and killed by allowing complete blood loss through the umbilical cord. The researchers incorrectly referred to their surgery as hysterectomy
, rather it was correctly a hysterotomy. The adult baboons were returned to the colony after recovery from the ordeal of major surgery. There was no mention or concern for the intense sense of loss all the mothers must have experienced as a result of having their babies taken from them.
Not surprisingly, as is well-documented (and acknowledged by the researchers), the mother baboons fed the high fat-high energy diet had greater body weights than those fed the control diet and the hearts of the dead foetuses, although outwardly normal, had some abnormal biochemical changes. The researchers stated the obvious, our data supports [sic] a growing dialogue around improved nutritional status prior to and throughout pregnancy and promotes [sic] the need for dietary interventions that will prevent adverse fetal metabolic programming
. What is particularly perplexing is that the researchers are currently undertaking long-term studies of human babies born to women on high-fat high-energy diets to track their health over decades, begging the question of why baboons are being subjected to experimentation and death.
This barbaric experiment, which resulted in substantial suffering for the mothers who had their babies taken away and killed, demonstrates once again the lack of humanity in the treatment of female non-human primates and their infants, as well as the lack of commitment to stop using non-human primates. Not only is the information in baboons irrelevant to people given the fundamental differences between the two species, humane and ethical clinical studies of the effects of diet have been and can continue to be done on human patients and volunteers in order to get data that are directly applicable to people. The reseachers of this baboon study cited many similar studies that have already been done in people. The baboons in this experiment suffered and their babies killed clearly contrary to the Replacement criterion for the 3Rs.
What you can do to help:
Cited information:
Information on NIH grant support (funding) is taken verbatim from relevant publications. If you have difficulty with any links provided, you can do your own search through the NIH RePORTER site: https://reporter.nih.gov/, by copying and pasting the grant number into the Search field on the form.
Be aware that some grants include funds for more than experiments on non-human primates.
9 September 2024: Macaques allowed to suffer and die in inhumane vaccine research in USA

Fifteen adult long-tailed macaques, whose home was in Asia, were purposefully infected with a lethal virus in order to see if they would survive after an experimental vaccine was used (Woolsey et al 2024. The work was done at the University of Texas Medical Branch in the USA and approved by their animal use committee. It was primarily funded by the public taxpayer through grants from the National Institutes of Health (NIH): AI094660, AI132223 and AI142790, totalling many millions of USA dollars.
The macaques, who were obtained from commercial suppliers PreLabs, Worldwide Primates and Charles River, were injected with a lethal dose of the Lassa virus. Three of the macaques were part of a 'pilot' study to see just how lethal was the dose used. All three had to be killed within 12 days because they were considered to have reached a humane endpoint
. Prior to reaching this humane endpoint
, the macaques were allowed to endure fever, decreased or complete loss of appetite, severe lethargy, hunched posture, weakness and seizures (signs of brain disease), as well as severe blood abnormalities – with no treatment to alleviate their suffering. Although the researchers stated that the macaques were euthanized
, this is a flagrant aberration of the meaning of euthanasia given that they were the ones who caused the macaques to reach such a degree of suffering that killing them was considered 'humane'.
Having developed a uniformly lethal cynomolgus monkey model
to study this strain of Lassa virus, the researchers then lethally infected 12 more macaques. After the macaques were showing signs of disease, ten received a vaccine and two were untreated. One of the untreated individuals died in 11 days, the other was killed on day 11 for humane
reasons. Three of the vaccinated individuals had to be killed within two weeks for humane
reasons. The rest of the vaccinated macaques were killed after several weeks.
Whether the experimental vaccine will have any beneficial effect in humans with Lassa virus infection is unknown. Studying humans who have survived this disease in order to learn more about the immunologic protection in them would provide more – and humane – useful information. As it was, the macaques in this study had to endure the stress of captivity, unalleviated suffering – often extreme – from a disease artificially induced in them, followed by death with only speculation as to the usefulness of the findings.
What you can do to help:
Cited information:
Information on NIH grant support (funding) is taken verbatim from relevant publications. If you have difficulty with any links provided, you can do your own search through the NIH RePORTER site: https://reporter.nih.gov/, by copying and pasting the grant number into the Search field on the form.
Be aware that some grants include funds for more than experiments on non-human primates.
13 August 2024: Infant snow monkeys killed to study human Western-style diet in USA research

Infant Japanese macaques (popularly known as snow monkeys) were used and killed in order to study the effects of a Western-style (calorically dense) diet on their pancreases at the Oregon National Primate Research Center in the USA (Carroll et al 2024). The work was approved by the institution's animal use committee and was paid for through public funds by grants from the National Institutes of Health (NIH) and its branches. The grants involved were primarily DK090964, DK128187, DK128416, DK135164, GM007347 and OD011092, totalling many millions of US dollars.
Eight adult female Japanese macaques were placed on either a typical Western-style diet or a standard monkey diet for at least two years before being placed in a situation where they could mate and become pregnant. They were sedated 2-3 times during pregnancy for fetal dating and third trimester measures. They were allowed to give birth naturally. The resulting eight offspring were given access to the same diet as their mother's and began eating it by four months of age, but remained with their mothers until forced weaning, which was around seven months of age and long before natural weaning would have occurred. After weaning, the infants were placed in social groups with similarly aged offspring and one to two adult females.
At 14 months of age, the offspring were killed (euphemistically referred to as humanely euthanized
by the researchers) using an anaesthetic followed by exsanguination (bleeding out) and tissue removed for further study.
What was learned? There were relatively minor differences in pancreatic cells between those on the Western diet and those on standard diet. None of this can be applied to humans, but the data can be obtained from appropriately done – and humane – studies on humans, as was documented by the researchers.
This brutal experiment, which resulted in premature death for the macaques, demonstrates the meaningless nature of the usual assurances the research industry offer the public about only using non-human primates when 'necessary'. Not only was the information learned through killing these infant macaques irrelevant to people given the fundamental differences between the two species, humane and ethical clinical studies have been and can continue to be done on human volunteers in order to get data that are directly applicable to people. There is no shortage of truly appropriate research subjects in the largest and most comprehensive laboratory: the world human population. There was no need for the researchers to concoct an inhumane experiment and waste intellectual and financial resources to get the information necessary for helping people. The infant macaques in this study had to endure the stress of captivity and death, and the mothers the loss of their infants, for what can only be considered frivolous reasons and clearly contrary to the Replacement criterion for the 3Rs.
What you can do to help:
Cited information:
Information on NIH grant support (funding) is taken verbatim from relevant publications. If you have difficulty with any links provided, you can do your own search through the NIH RePORTER site: https://reporter.nih.gov/, by copying and pasting the grant number into the Search field on the form.
Be aware that some grants include funds for more than experiments on non-human primates.
10 August 2024: Monkeys destined for Charles River testing lab stranded at airport for 27 hours

Hundreds of Endangered long-tailed macaques were temporarily stranded at Tbilisi International Airport for over 27 hours on 9-10th August 2024. The monkeys, destined for Charles River testing laboratory in Montreal, were being transported from Cambodia to Canada by SkyTaxi. The flight, due to land at Montreal Mirabel International Airport, was initially denied entry by the Canadian Transportation Agency after it was discovered SkyTaxi was in violation of its licence. This discovery followed a complaint submitted to the Canadian authorities by Abolición Vivisección.
The trade, transportation and use of non-human primates by the global research and toxicity (poisoning) testing industry is responsible for extreme suffering and death. The trauma and distress experienced by monkeys during this flight alone is unimaginable. When finally arriving in Montreal, these monkeys will have endured over 45 hours imprisoned singly in small transit crates: an eight hour flight from Cambodia, stranded for 27 hours on the ground at Tbilisi airport and then an 11 hour flight to Montreal. This does not include the additional hours of ground transportation from the breeding facility to the airport and from the destination to the laboratory.
SkyTaxi has transported thousands of Endangered long-tailed macaques from Mauritius, Cambodia and Vietnam to non-human primate dealers and testing laboratories in the USA, Canada and Europe.
Action for Primates has joined with People for the Ethical Treatment of Animals, Abolición Vivisección, Cheshire Animal Rights Campaigns and One Voice in calling on the Canadian authorities to stop all SkyTaxi shipments of monkeys, seize those already in Canada and send them to accredited sanctuaries.
What you can do to help:
2 August 2024: Macaques subjected to brain damage and death to study human paranoia

In this disturbing experiment, 20 adult male rhesus macaques were used across three laboratories in order to see the effects of damaging certain parts of their brains on their behaviour as part of research attempting to study paranoia in humans (Suthaharan et al 2024). Although the researchers involved listed many affiliations from several countries, the work was apparently done at the University of Oxford in the UK and the National Institute of Mental Health (NIMH) in the USA, and was approved by their respective animal use committees. The primary funding appeared to be from grants through the NIMH: MH112887, MH120081, MH120799 and MH128190, totalling many millions of US taxpayer dollars.
Highly invasive brain surgery (craniotomies) was done to cause damage to two regions of the brain (in different macaques in order to compare responses). The damage was cause by injecting toxins into brain tissue or by cutting into the brain. Some of the macaques underwent several such surgeries. In addition to the macaques who were subjected to surgery, other macaques were used as unoperated controls
.
The macaques were tested in a cage in front of a touch screen. Sessions involved 300 trials with food pellets used as rewards for the desired response. At least some of the macaques were killed in order to get brain tissue to be sure the intended lesions had occurred subsequent to the surgeries. What happened to the others was not stated.
Human volunteers were also used – 1,225 of them – and were tested similarly to the macaques. This begs the question of why, if the essential information could be gained from humans, the macaques were subjected to such a brutal experiment. The researchers themselves appeared to indirectly question this when they made the statement, It is controversial whether it is feasible to model psychiatric symptoms like paranoia in nonhuman animals.
Further, the researchers documented many studies on humans with paranoia and recognised that pivotal information can be derived particularly from humans with naturally occurring or acquired brain damage in specific regions of the brain, similar to those artificially created in the macaques. The situation for the macaques (caged under unnatural, laboratory conditions and mechanically responding in order to obtain food) is completely different to the complex variables that influence the human situation.
Once again, non-human primates are being subjected to inhumane and highly invasive brain surgery in order to study a phenomenon that can only be studied effectively in humans. This is clearly contrary to the Replacement criterion for the 3Rs, to which these laboratories say they adhere.
What you can do to help:
Cited information:
Craniotomy:
This is a surgical procedure to access the brain. Typically, an area on the head is shaved. An incision is made through the skin and tissues underlying it, down to the surface of the skull, in such a way as to create a flap that can be pulled away to expose the skull. Depending on what type of access to the brain is required, holes may be drilled through the skull into the brain cavity, for example for injections, or a portion of the bone may be cut out in order to implant a bank of electrodes. When done, bone cement may be used to fill in the hole in the skull and the overlying tissues sutured.
Information on NIH grant support (funding) is taken verbatim from relevant publications. If you have difficulty with any links provided, you can do your own search through the NIH RePORTER site: https://reporter.nih.gov/, by copying and pasting the grant number into the Search field on the form.
Be aware that some grants include funds for more than experiments on non-human primates.
9 July 2024: Macaques forced to inject cocaine in publicly funded research in USA

Female and male long-tailed macaques were used to determine the effects of social status on the use of cocaine (Johnson et al 2024). The work was done at Wake Forest University and approved by their animal use committee and purportedly performed per the NIH Guide. It was funded by the USA taxpayer through grants from the National Institutes of Health: DA017763, DA041349 and DA053776, totalling several million US dollars.
The goal of this experiment was to see the effects of social status on cocaine self-administration
. Nine female and six male long-tailed macaques were used. They were housed in all female or all male groups in very small stainless steel cages (0.71 x 1.73 x 1.83 metres), but were partitioned so that each macaque was in an even smaller compartment (only 0.71 x 0.84 x 0.84 metre) separated from the others by a partition. They were not allowed complete social contact except when the partitions were removed. Each macaque was fitted with an aluminium neck collar and restrained in a sitting position in a device euphemistically called a 'primate chair' produced by Primate Products. Although it was not explicitly stated, the reason for fitting macaques with neck collars is so that poles can be attached to the collars and the macaques pulled from their cages and forcibly moved around the laboratory, including to be confined in 'chairs'.
The macaques were 'trained' to use a device in order to receive flavoured food pellets while sitting in an isolation sound-proof chamber large enough to accommodate the chair in which they were restrained. They were subjected to surgery to implant a catheter (tube) into a major vein (femoral or internal or external jugular). The catheter was 'tunnelled' under the skin all the way to about the middle of the back where another incision was made to create a 'pocket' into which a pump was implanted. This setup was to allow the macaques to inject cocaine directly into their bloodstreams once they were 'hooked' on it.
The source of the macaques or their fate after the experiment was finished was not stated. Nor was there information on what cocaine did to the daily lives of the macaques or whether they suffered from any kind of withdrawal when the cocaine was stopped.
What was 'learned'? Under the completely artificial conditions of the experiment, the data in females suggests [sic] that duration of social enrichment and stress can differentially impact vulnerability to cocaine reinforcement
and in males the data confirmed earlier social-rank differences using an FR [fixed-ratio] schedule and showed that vulnerability could be modified by exposure to cocaine
. None of this has any relevance to humans suffering from the disease of addiction. The data to help these individuals are already available and more can be derived from humane and human-relevant investigations using humans with substance abuse issues. Other researchers have been critical of the use of non-human primates to 'model' the human with substance abuse disorder, pointing out what we have been saying for years: addiction in humans is a complex disorder which encompasses psychological, social and emotional variables that are impossible to model in animals
(Magliaro & Ahluwalia 2022). It is unconscionably naive to keep subjecting non-human primates to a mechanistic approach in a quest to help people. Why are non-human primates being forced to consume cocaine in cruel and crude experiments for a disorder that is unique to humans?!
What you can do to help:
Cited information:
...the impact of social, psychological and economic factors that determine the onset and persistence of addiction (and recovery from it) in humans. Indeed, the predictive validity of animal models of addiction is poor, with few effective treatments deriving from animal studies. ...the principal argument against animal experiments in the field of substances of abuse is that addiction in humans is a complex disorder which encompasses psychological, social and emotional variables that are impossible to model in animals.
Pole and collar:
This refers to placing a permanent metal collar around the neck of the individual. The collar has openings which can be 'grabbed' by the end of a specially designed pole. The individual then can be dragged around by the neck until they acquiesce to being manoeuvred in this manner. Almost all individuals vigorously object to being treated in this manner and it can take many weeks for them to acquiesce. Some become too aggressive for this method to be used.
Primate chairs:

So-called 'primate chairs', such as those manufactured by Crist Instrument Co, are restraint devices which secure the individual in an unnatural sitting position. Further restraint is often used through a neck plate which goes around the neck to prevent side-to-side or front to back movements of the head. The head may be severely restrained through the use of a post surgically implanted into the skull and held by part of the chair. Depending on the procedures being done, the arms and legs may be secured further by taping them to the chair supports.
Information on NIH grant support (funding) is taken verbatim from relevant publications. If you have difficulty with any links provided, you can do your own search through the NIH RePORTER site: https://reporter.nih.gov/, by copying and pasting the grant number into the Search field on the form.
Be aware that some grants include funds for more than experiments on non-human primates.
30 June 2024: Urge SkyTaxi to stop transporting monkeys

Action for Primates is urging SkyTaxi, the Polish charter airline, to stop the transportation of monkeys for the research and toxicity (poisoning) testing industry. Information uncovered from various sources has revealed that SkyTaxi is now regularly transporting thousands of Endangered long-tailed macaques from Mauritius, Cambodia and Vietnam to non-human primate dealers and testing laboratories in the USA, Canada and Europe. Their fate at their destinations will be more suffering, eventually to be killed.
During the week of 24 June 2024, SkyTaxi transported hundreds of long-tailed macaques from Mauritius to Houston, USA, via Brussels. The aircraft then flew from Houston to Vietnam to load up hundreds more monkeys – destined for Charles River Laboratories – and transported them to Washington Dulles Airport, USA. Each flight involved a 26 hour plus ordeal, including a six hour stop-over.
On 16th June 2024, SkyTaxi transported hundreds of long-tailed macaques from Mauritius to the USA via Brussels. The monkeys were subjected to over 28 hours in the cargo hold of the SkyTaxi aircraft, including a six hour stop-over in Brussels.
In April 2024, the airline loaded long-tailed macaques from Mauritius to transport to Brussels, and then flew on to Cambodia to load more monkeys to transport to Canada. From Cambodia to Montreal, the monkeys were subjected to an arduous 26-hour ordeal in the cargo hold, including a five and a half hour stop-over en route. The monkeys were destined for Charles River Laboratories.
The transportation by airlines of non-human primates destined for research and toxicity testing is part of a brutal global industry. Torn from their families and friends, these intelligent, sentient and complex beings are forced to endure a traumatic and frightening ordeal, each confined to a small, single crate. They are subjected to unimaginable stress and anxiety as they are loaded into cargo holds and exposed to the bewildering and frightening unnatural sounds and sensations of air travel thousands of miles across the world.
As a result of public concern and opposition, many airlines and cargo carriers, including American Airlines, British Airways, United Airlines, South African Airways, Delta Airlines, Eva Air, Air Canada, China Airlines and Kenya Airways, made the decision to end their involvement in the cruelty and suffering caused by the international trade in non-human primates, by refusing to transport these animals destined for the research industry. More recently, Air France stopped in 2023, after decades of transporting long-tailed macaques from Mauritius and Vietnam to the USA and Europe.
Please join us in calling on SkyTaxi to do likewise, and to place a permanent embargo on the transportation of monkeys:
24 June 2024: Pregnant rhesus macaques fed cannabis, near-term foetuses removed and killed

US tax payers and not-for-profit organisations paid for a wasteful and cruel experiment forcing rhesus macaques to consume tetrahydrocannabinol (THC) to see its effects on neurodevelopment in foetuses (Ryan et al 2024). Tetrahydrocannabinol is the principal psychoactive constituent of cannabis. The experiment was carried out at the Oregon National Primate Research Center in Oregon, USA. It was approved by their animal use committee and reportedly conformed with all guidelines for humane animal care. Funding was through the National Institutes of Health grants AA007468, AA028769, DA056493, HD097116 and OD011092, awards totalling many tens of millions of US dollars. Additional funding was provided by the March of Dimes, the Silver Family and the Society for Maternal-Fetal Medicine.
Ten indoor-housed adult female rhesus macaques were used as surrogates for pregnant humans. Despite claiming to conform to ARRIVE guidelines, the researchers provided no further information about housing, whether there was any social or other enrichment. Information such as this is important, not only in terms of welfare, but also to evaluate the scientific credibility of the work (Pomerantz et al 2022). Five of the macaques were fed THC, the other five received no drug (controls
). All were subjected to anaesthesia and MRI (magnetic resonance imaging) throughout the study.
On gestational day 155, the mothers were subjected to caesarean section, but no details were provided on how this was done. The foetuses were removed and killed to study their brains. There was no mention of the impact on the mothers who were deprived of their foetuses, who were near term. Nor was there mention of the impact of the THC on the daily lives of the mothers. Removing and killing unborn infants – foetuses who were almost (if not at full-term, the gestation period being 146-180 days) – was despicable and demonstrates an utter lack of humanity.
None of the information gained from this experiment can uncritically be applied to the human situation. More importantly, as well-documented by the researchers, the essential information to counsel and help human mothers and children concerning the developmental effects of THC is already available and humane and human-relevant studies can continue to improve the health of women and children.
The public are repeatedly told that non-human primates are used in research only when absolutely necessary and only when there are no other alternatives available. This despicable experiment, which resulted in substantial suffering for the macaques and death for the foetuses, demonstrates the meaningless nature of such assurances and a lack of commitment to stop using non-human primates. Other researchers in the field of human substance abuse argue that the 'animal model' is not only inhumane, but not scientifically credible and that very little can be or has been learned to help people (Magliaro & Ahluwalia 2022).
What you can do to help:
Cited information:
recommendations for the full and transparent reporting of research involving animals – maximising the quality and reliability of published research, and enabling others to better scrutinise, evaluate and reproduce it
...the impact of social, psychological and economic factors that determine the onset and persistence of addiction (and recovery from it) in humans. Indeed, the predictive validity of animal models of addiction is poor, with few effective treatments deriving from animal studies. ...the principal argument against animal experiments in the field of substances of abuse is that addiction in humans is a complex disorder which encompasses psychological, social and emotional variables that are impossible to model in animals.
Information on NIH grant support (funding) is taken verbatim from relevant publications. If you have difficulty with any links provided, you can do your own search through the NIH RePORTER site: https://reporter.nih.gov/, by copying and pasting the grant number into the Search field on the form.
Be aware that some grants include funds for more than experiments on non-human primates.
28 May 2024: Healthy rhesus macaque infants killed in maternal obesity study

Pregnancy complications for women who are obese include development of gestational hypertension and pre-eclampsia. In this experiment, researchers used nineteen pregnant female rhesus macaques as surrogates for obese, pregnant female humans in order to see if caloric restriction or the use of a compound already in use in human mothers made a difference. The macaques were from an indoor breeding colony at the California National Primate Research Center in Davis, California (Hasegawa et al 2023). The research was approved by the UC Davis animal use committee and was paid for using public funds (NIH grants HD084203, HD103526, OD010962, OD011107; USDA grant 1021411).
The mothers for the test group were ones who were considered to be obese
, had been successful in producing offspring in the past and who were pregnant with a male foetus. A similar group of mothers who were not obese were part of the lean control
group. A total of 19 obese mothers were assigned to either one of the two intervention groups: five received a restricted supply of food; seven received pravastatin, a statin drug already in use in humans; seven comprised an untreated obese control group. Maternal gestational samples and postnatal infant samples were compared with six lean control mothers. The researchers euphemistically referred to the mothers as being enrolled
, as if the mothers had a choice.
Although most of the macaques were allowed to deliver naturally, cesarean delivery was done when it was believed that there would be problems otherwise. Following the surgery and birth, the mothers of these infants did not accept them, and the infants had to be fostered, adding to their stress.
The infants were subjected to a series of tests to determine their brain and behavioural development. To collect samples, anaesthesia using ketamine or Telazol was used. It is well-known that chemical restraint is associated with discomfort at best, particularly during recovery.
All infants were killed (euphemistically and incorrectly referred to as euthanasia
by the researchers), apparently at 180 days of age. They were first fasted (how long not stated in Methods), then anaesthetised. After collection of samples, they were killed and then their kidneys and brains were removed for evaluation. There was no mention or apparent concern for the extreme distress the mothers experienced as a result of the loss of their infants.
What was learned? Although the two interventions tested led to some improvements in metabolism or infant brain development, negative impacts were also found in the mothers and infants. Nevertheless, the researchers stated that their study deepened the understanding of the impacts of calorie restriction or pravastatin administration in mothers with obesity on maternal and offspring health.
It should be obvious to anyone, that the results were only valid for rhesus macaque mothers, that the deepened...understanding
had nothing to do with human mothers. Furthermore, the results were substantially confounded because the rhesus macaques were in captivity where life is far from normal. It is well known that translatability between artificial studies in non-human primates to humans is tenuous at best and dangerous at worst.
Obesity is one of the world's greatest health problems, with more than a billion people globally now considered to be obese. As well documented in the paper by the researchers, there are already substantial data on the impact of obesity on reproductive and developmental health in humans. It is morally repugnant that pregnant monkeys are being used in this cruel research, with their babies, at just a few months old, taken from them and killed. There is no reason why well-designed, humane and human-relevant studies cannot continue to be done on the best and largest laboratory in the world: the human population.
What you can do to help (for more information on the issue, see below):
Cited information:
Euthanasia:
The term euthanasia means that an individual is being killed by another, but solely with the interests of the individual dying in mind. Of necessity, this means that the individual dying would benefit from death by ending a situation that is causing intractable suffering. Ideally, the individual would be able to indicate that he or she prefers death to continued life. In some situations, particularly in veterinary medicine, this may not be feasible because of an inability to communicate with the individual. In these situations, it becomes especially important that the person ending life must be clear on their motives which must derive only from a sincere belief that ending the life is in the best interests of that individual at that moment. If the person is also the one who placed the individual in a situation causing suffering, as is usually the case in a research setting, it would be a perversion of the definition to label this as 'euthanasia'. One cannot get credit for ending suffering for which they are responsible.
In addition to considering the interests of the individual being killed, euthanasia also demands that the method of death be as quick, painless and stress-free as humanly possible. Any method that results in more than momentary and mild pain, creates an atmosphere of extreme anxiety or otherwise impacts negatively on the individual cannot logically be considered euthanasia.
Information on NIH grant support (funding) is taken verbatim from relevant publications. If you have difficulty with any links provided, you can do your own search through the NIH RePORTER site: https://reporter.nih.gov/, by copying and pasting the grant number into the Search field on the form.
Be aware that some grants include funds for more than experiments on non-human primates.
13 May 2024: Rhesus macaques used in disturbing research in Europe to 'learn' their responses to music

Contrary to all the norms of using non-human primates in research only when absolutely necessary and only when there are no other alternatives available, rhesus macaques who had head posts and brain electrodes implanted from a previous experiment, were subjected to severe restraint to see how they responded to music (Bianco et al 2024). The research was apparently done at the Italian Institute of Technology, with additional researchers affiliated to institutions in Denmark, the Netherlands and the UK (see list). Funding included public and private sources of funds (funding sources). Animal care and housing, and the experimental procedures were stated to have complied with European (EU Directive 63-2010) and Italian (DL. 26/2014) laws on the use of non-human primates in research.
Two adult male rhesus macaques were euphemistically referred to as participants
, as if they had made a conscious choice to be used in experimentation. The macaques had been experimented upon previously by several of the Italian researchers in the current group (Novembre et al 2024), during which they were subjected to invasive surgery to implant a head post into their skulls and electrodes into their brains through a craniotomy). They were restrained in a Crist Instrument Co primate chair). Their heads had been shaved for electroencephalogram electrodes.
In the present experiment, the macaques were forced to endure 26 sessions with their heads severely restrained using the head posts in dark, sound-proofed room facing a screen. Each session lasted at least 95 minutes. The macaques were forced to listen via two audio speakers to music (piano and musical melodies) at about 75 dB. Their responses, using a computer joystick, were recorded as timing and pitch changed. Their fate after the experiments were concluded was not stated.
Startlingly, the researchers had already available the same data from human volunteers, who had made a conscious choice to be involved and did not have to be subjected to brutal head surgery. This meaningless experiment, which resulted in substantial suffering for the macaques, demonstrated once more that researchers and licensing authorities are insincere when they try to assure the public about only using non-human primates when necessary. It is unquestionable that there is a lack of commitment to stop using non-human primates. Not only is the information in macaques irrelevant to people given the fundamental differences between the two species, humane and ethical clinical studies have been and can continue to be done on human patients and volunteers in order to get data that are directly applicable to people. Learning how macaques respond to music is certainly not consistent with the public pronouncement by the Italian Institute of Technology: We strive to excel in basic and applied research in order to address the big challenges of humanity
. The macaques in this study had to endure the stress of captivity, earlier major survival surgery and severe restraint for what can only be considered frivolous reasons and clearly contrary to the Replacement criterion for the 3Rs.
What you can do to help:
Cited information:
Craniotomy:
This is a surgical procedure to access the brain. Typically, an area on the head is shaved. An incision is made through the skin and tissues underlying it, down to the surface of the skull, in such a way as to create a flap that can be pulled away to expose the skull. Depending on what type of access to the brain is required, holes may be drilled through the skull into the brain cavity, for example for injections, or a portion of the bone may be cut out in order to implant a bank of electrodes. When done, bone cement may be used to fill in the hole in the skull and the overlying tissues sutured.
Primate chairs:

So-called 'primate chairs', such as those manufactured by Crist Instrument Co, are restraint devices which secure the individual in an unnatural sitting position. Further restraint is often used through a neck plate which goes around the neck to prevent side-to-side or front to back movements of the head. The head may be severely restrained through the use of a post surgically implanted into the skull and held by part of the chair. Depending on the procedures being done, the arms and legs may be secured further by taping them to the chair supports.
Head posts:
As the name implies, these are posts that are attached to the skull. Surgery is done on the head to dissect through skin and other tissues down to the surface of the skull. The post is cemented or screwed in place. Once healing has occurred, the post is used to restrain the head while recordings or other experimentation is done. It is almost always combined with a neck plate so that the non-human primate cannot move their heads.
Affiliations included Neuroscience of Perception & Action Lab, Italian Institute of Technology, Italy, the Center for Music in the Brain, Department of Clinical Medicine, Aarhus University & The Royal Academy of Music, Denmark, and the Department of Psychology, Nottingham Trent University, UK.
Individual researchers were funded by the European Union and the European Research Council, Center for Music in the Brain was funded by the Danish National Research Foundation and The Comparative Bioacoustics Group was funded by Max Planck Group Leader funding.
5 May 2024: Captive Primate Safety Act reintroduced in USA Congress

Action for Primates has welcomed the reintroduction in Congress of the Captive Primate Safety Act in the US by Representative Earl Blumenauer (D-OR), Representative Brian Fitzpatrick (R-PA) and Senator Richard Blumenthal (D-CT). This bipartisan legislation, introduced on 1 May 2024, would ban the private 'ownership' of all non-human primates as 'pets', and restrict the public having direct contact with non-human primates.
Senator Blumenthal stated: Wild animals belong in the wild, not shackled and mistreated in someone's backyard. Humans often are injured by wild animals kept as pets because their deeply ingrained instincts resist domestication, causing them to be dangerously unpredictable pets. The Captive Primate Safety Act is about safety, but also basic humane behavior– ending exploitation of these human-like, highly intelligent, social animals.
In the US, the private 'ownership' of non-human primates is still legal in many states. These animals are bred commercially to be sold as 'pets'; infants are removed from their mothers and advertised for sale dressed in human children's clothes. They may even have their tails removed to make it easier to put on diapers. Non-human primates are wild animals and do not belong in captivity in homes and backyards. They should be living freely with their family and social groups in their native habitat. Depriving them of their freedom, the companionship of others of their kind and keeping them under totally unnatural conditions, is cruel and immoral.
When kept in captivity as 'pets', non-human primates can become a danger to people. As they mature and become physically stronger, they become unpredictable and aggressive towards humans and can cause serious injuries. To deal with this aggression, people will often add to the inhumanity of the situation by removing teeth and nails in the erroneous belief that this will prevent injuries.
You can find more information on the issue of non-human primates in private homes on our Non-human Primates in Private Homes ('Pets') page.
This is a critical piece of legislation and the fact that it has bipartisan support is monumental. If you are a US citizen, please contact your president and federal legislators and politely urge them to support this bill. We need to show Congress that the public do not want to continue the vile practice of imprisoning non-human primates as 'pets'.
Use this link to find your legislators: https://www.usa.gov/elected-officials
29 April 2024: Appeal to Ethiopian Airlines to stop transporting monkeys

Action for Primates is appealing to Ethiopian Airlines to end its involvement in the transportation of monkeys for the research and toxicity (poisoning) testing industry. The appeal follows an alert received by Action for Primates that Ethiopian Airlines recently transported 250 long-tailed macaques, originating in Mauritius, to the USA. The monkeys were initially transported by Safe Air, a Kenyan based cargo airline, to Addis Ababa, before being transferred to an Ethiopian Airline's aircraft for onward transport to the USA.
The long-tailed macaques were exported by the Mauritius monkey dealer, Bioculture, to BC US LLC, its facility in Florida. Bioculture is a major exporter of macaques, including those captured in the wild. In 2022, the company exported over 4,000 wild-caught monkeys to animal supply and testing laboratories in the USA.
The transportation by airlines of non-human primates destined for the research and toxicity testing industry is an issue that invokes strong public concern. These intelligent and sensitive animals are subjected to unimaginable stress and anxiety as they are packed into small crates and loaded into cargo holds, and exposed to the bewildering and frightening unnatural sounds and sensations of air travel thousands of miles across the world. Many airlines have ended their involvement in this cruel business, including Air France which stopped in 2023, after decades of transporting long-tailed macaques from Mauritius to the USA and Europe.
Please join us in calling on Ethiopian Airlines to do likewise, and place a permanent embargo on the transportation of monkeys:
15 April 2024: Rhesus macaques irradiated and allowed to suffer in publicly funded research in USA

Female and male adult rhesus macaques were subjected to lethal irradiation by several USA governmental agencies including the Naval Medical Research Center and the Uniformed Services University of the Health Sciences, as well as the University of Arkansas for Medical Sciences (Singh et al 2024). The experiment was done at BIOQUAL, Inc, a contract testing laboratory in Maryland. The work was funded by the USA taxpayer through grants by Congress and the Armed Forces. According to the researchers, the procedures were approved by the animal use committee of BIOQUAL and the Department of Defense.
Thirty-two rhesus macaques (18 females and 14 males) were used for this study. They were between 3.5 and 5.5 years old. The researchers claimed to follow the 3Rs by using only eight macaques per experimental group.
The macaques were all housed singly and were denied direct contact with each other, something that is critical to their well-being. They were anaesthetised and subjected to either partial or total body irradiation at doses expected to cause death. The purpose was to study the damage to various organs and to see if a particular treatment called GT3 might have a beneficial effect. The researchers had planned to kill three macaques in each group on days four and seven, and the remaining animals on day ten post-irradiation for tissue collection. By day seven post-irradiation, however, all the macaques were moribund [at the point of death] and were killed, according to the researchers, to save them from pain and suffering
. Before the macaques reached a moribund state, however, they would have suffered substantially as a result of radiation poisoning. The researchers only alluded to this by pointing out that the macaques developed severe injuries including functionally crippling lesions within various major organ systems
. The tested treatment had limited protective effect.
This was an extremely inhumane experiment. It is morally reprehensible that these monkeys were used as surrogates for humans and made to suffer unimaginably as a result of being poisoned with irradiation. We already know that irradiation causes major organ damage, suffering and death in humans. It is morally unconscionable to cause similar suffering and death in others.
What you can do to help:
Reference:
9 April 2024: Join call to USA to end cruel macaque imports from Indonesia

Action for Primates and Lady Freethinker are calling on the USA to stop importing long-tailed macaques from Indonesia. The call follows the revelation that, in 2023, over 1,400 long-tailed macaques, who were captured from the wild in Indonesia, were imported by the USA research and toxicity (poisoning) testing industry. This was despite widespread global concerns about the inherent inhumanity of trapping wild monkeys and increasing awareness of the vulnerability of the conservation status of this species.
During 2023, there were three shipments of long-tailed macaques: 322 individuals imported on 17th May; 540 on 31st May and 540 on 27th December. All the monkeys were reported as having been captured in the wild. This represented an increase of almost 40% since 2022, when 870 wild-caught macaques were imported by Primate Products in Florida, and 120 captive born macaques were imported by Charles River Laboratories.
Action for Primates has previously released harrowing video footage of the capture of wild long-tailed macaques in Indonesia. This footage provides compelling evidence of the cruelty of the trappers and the suffering and distress caused to the monkeys. The cruelty included brutal capture methods, the forced separation of nursing infants from their mothers and the beating and killing of unwanted individuals. Such inhumane treatment is a clear breach of international animal welfare guidelines.
Long-tailed macaques are CITES listed Appendix II, with their conservation status having been increased to Endangered with a decreasing population trend by the International Union for the Conservation of Nature (IUCN) Red List of Threatened Species.
The USA is one of the world's major importers and users of non-human primates. Long-tailed macaques are the primary non-human primate species used in regulatory toxicity tests, which is the area in which most non-human primates are used. Toxicity (or poisoning) testing is carried out to assess adverse reactions to drugs (or chemicals), and often involves substantial suffering and death.
Nina Jackel, Founder of Lady Freethinker, stated: The spike in macaques being shipped from Indonesia to the U.S. for research and testing purposes is shocking and alarming. The evidence clearly shows that these animals are suffering immensely, and it's time for the government to take meaningful action to stop the cruelty.
Sarah Kite, co-founder, Action for Primates, stated: Capturing monkeys from the wild inflicts substantial suffering. The handling and treatment of the monkeys seen in the Action for Primates video is brutal and inherently inhumane. It is also a clear breach of international animal welfare guidelines. Action for Primates is urging the USA government to dissociate itself from this extreme cruelty by banning all imports of monkeys from Indonesia.
Please join our call by signing the Lady Freethinker and Action for Primates petition urging the United States government to immediately end the import of monkeys from Indonesia:
https://ladyfreethinker.org/sign-justice-for-indonesian-monkeys-trapped-and-brutalized/
Long-tailed macaques are indigenous to Indonesia, part of the rich and diverse ecosystem, contributing to the country's unique biodiversity. The species, however, is not protected under Indonesian law and, in addition to the capture and export for the global research and toxicity testing industry, its wild populations face many other threats, including hunting for human consumption; capture as 'pets' or to be used in tourism and 'entertainment' activities, including the disturbing rise in baby macaque abuse videos filmed for broadcast on social media; and killing due to negative interactions with people.
What you can do to help:
18 March 2024: Concerns over slaughter of green monkeys in Barbados

Green monkeys are not indigenous to Barbados. They were introduced to the island, and several other countries in the Caribbean, by humans many hundreds of years ago. As a result of a highly favourable climate, feeding opportunities and no major natural predators, the species has increased in numbers since. Because the human population has increased and habitat has been destroyed in order to grow human crops, there is competition for resources between the monkeys and humans.
Rather than deal with this issue humanely and effectively, the Barbados government has decided to indiscriminately slaughter monkeys in a short-sighted attempt at mitigation – offering a bounty of $25 per monkey to farmers to hunt the animals. The slaughter of potentially hundreds of sentient and intelligent beings is inhumane and unacceptable. Further, it is well-known that killing monkeys in an attempt at 'population control' fails because of the rebound increase in reproduction caused by this. Removing them by the thousands has been shown to also fail (Boulton et al 1996).
Whereas we understand the concerns of the farmers and those people producing human food, we know that this proposed 'quick fix' will simply result in more killing on a regular basis. It would be far more efficient – and humane – to develop a comprehensive, well-managed sterilisation scheme. Although this will not immediately reduce monkey numbers, it will have a lasting effect that can keep numbers in control in the long term. In the meantime, there are numerous measures that can be taken to humanely reduce some of the unfavourable interactions with people (Springer 2020). The study by Springer was commissioned as a result of recommendation by The African Green Monkey Sub-committee of The Biodiversity Conservation and Management Section of The Ministry of Environment and National Beautification, Barbados. We do not know how much, if any, of the recommendations in the manual have been implemented. It is, nevertheless, alarming that Barbados is promoting extermination rather than an effective, long-term solution such as sterilisation combined with deterrence.
What you can do to help:
Reference:
12 March 2024: URGENT appeal – help to ban 'trophy' hunting imports into Great Britain

On 22nd March, the UK Government has another opportunity to deliver on its manifesto commitment to ban the import into Great Britain of body parts from endangered and vulnerable wild animals, including non-human primates such as baboons, green and other monkeys, hunted and killed overseas for 'sport' and 'entertainment', so-called trophies.
Action for Primates, along with other animal and wildlife protection groups as part of the Coalition Against Trophy and Canned Hunting (CATCH), wrote to the Secretary of State for Environment, Food and Rural Affairs. We urged the Government to remain committed to deliver on its manifesto pledge and to ensure the Hunting Trophies (Import Prohibition) Bill attains Royal Assent.
A ban on the import of these sickening 'trophies', including skins, severed heads, other body parts and carcasses, was promised in the 2019 manifesto by the Conservative Party. Despite widespread public and cross-party political support, in September 2023, the Hunting Trophies (Import Prohibition) Bill by Henry Smith MP was deliberately blocked by a small minority of pro-hunting peers in the House of Lords.
Henry Smith recently told the Daily Express newspaper: Sadly, the bill was killed off by a small group of Lords when it reached their House...A ban was promised in the 2019 manifesto that Conservative MPs such as myself were elected on and is strongly supported across the political spectrum in the Commons. For it to have been thwarted by a minority peers was a terrible denial of democracy and shocked many people.
The good news is that an identical Hunting Trophies (Import Prohibition) Bill is now being led by John Spellar MP, and is due to have its Second Reading in the House of Commons on Friday 22nd March.
It is sickening that these wonderful, sentient beings living freely in the forests and savannas of Africa, have their lives cruelly taken from them so that their bodies can end up as 'trophies' on someone's wall. If you are a UK citizen, please urgently contact your MP asking them to attend Parliament on Friday 22nd March and to vote for the Hunting Trophies (Import Prohibition) Bill by John Spellar MP.
Here is sample wording you can copy, edit and send as a message to send to your MP:
Dear I am writing to you regarding an important Bill that receives its Second Reading in the House of Commons on Friday 22nd March 2024, a Bill that would ban the import of hunting 'trophies'. Wild endangered animals, including lions, rhinos, giraffes and monkeys are being hunted and slaughtered by people from the UK for 'sport' and 'entertainment'; their bodies dismembered and imported into the country as 'trophies'.
There is widespread political support for such a ban. In September 2023, a similar Bill from Henry Smith MP received widespread support in Parliament, but was deliberately blocked by a small minority of pro-hunting peers in the House of Lords.
I would be very grateful if, as my Member of Parliament, you will attend Parliament on 22nd March and vote for John Spellar's "Hunting Trophies (Import Prohibition) Bill" to end this cruel and shameful trade in endangered animals' body parts.
Sincerely,
You can find your MP here: https://members.parliament.uk/FindYourMP
5 March 2024: Squirrel monkeys deliberately subjected to spinal cord injury and death in public funded research in USA

Guianan squirrel monkeys at Vanderbilt University in Tennessee, USA, have been mutilated and killed in an attempt to replicate spinal cord injury in humans (Manzanera Esteve et al 2024). Injury of this type results in serious and permanent loss of body movement and sensation depending on the location and degree of damage. The work was approved by the University's animal use committee and funded by the USA taxpayer through grants NS092961 (millions of $ US) from the National Institute of Neurological Disorders and Stroke and SC160154 (about $783,000 US) from the Department of Defense.
Four adult male Guianan squirrel monkeys were used, source not stated. After determining each monkey's dominant hand use, the monkeys were anaesthetised and subjected to deliberate injury to their spinal cord. The C5 site (neck region) was cut through in order to prevent the monkeys being able to use or feel fine touch sensations from the fingers of the dominant hand. This required subjecting the monkeys to a laminectomy (the removal of part or all of the vertebral bone to expose the spinal cord) after cutting through skin and other tissues. The wounds were sutured closed and the monkeys were returned to their cages after they awakened. A negative impact on hand use was reported by the researchers: all animals displayed hand use impairments, characterized by an unwillingness to use the injured hand for cage climbing, abnormal hand posture while holding food, and an increased number of false positive attempts in food retrieving trials
. What the researchers failed to report was the psychological impact to the monkeys. It is reasonable to assume that the monkeys would have experienced anxiety and confusion by what had happened to them.
The monkeys were repeatedly tested for their ability to grasp and retrieve food. They were also anaesthetised numerous times over the next 24 weeks in order to do MRI scans. Recovering from anaesthesia involves considerable suffering. When the experiment was over, all the monkeys were killed (euphemistically referred to as euthanized
by the researchers) to get tissue for further study.
The conclusion by the researchers was that this methodology could be utilized to guide clinical treatments aimed at reducing the extent of injured tissue and enhancing the recovery mechanisms
. No cure, no treatments, no progress in terms of directly helping people with spinal cord injuries. This was a highly technical and basic science approach to a very specific type of cervical spinal cord injury in one species of non-human primate. This may have no relevance for other primate species, including humans. This kind of reliance on animal 'models' has even been criticised by a previous NIH Director (McManus 2013). Moreover, we know that basic science research may not provide any clinical insight (Contopoulos-Ioannidis et 2003).
There are thousands of human patients with spinal cord injuries in the USA alone. The kind of information derived by artificially mutilating the spinal cords of monkeys could easily be obtained using such human patients with different types of spinal cord injury, providing ethical, humane and human-relevant data. It is this kind of information that will ultimately be of direct use in helping human patients.
The public are repeatedly told that non-human primates are used in research only when absolutely necessary and only when there are no other alternatives available. This barbaric experiment, which resulted in substantial suffering and death for the monkeys, demonstrates the meaningless nature of such assurances and a lack of commitment to stop using non-human primates.
What you can do to help:
Cited information:
[Elias A. Zerhouni, M.D., NIH Director 2002-2008]: "We have moved away from studying human disease in humans," he lamented. "We all drank the Kool-Aid on that one, me included." With the ability to knock in or knock out any gene in a mouse—which "can't sue us," Zerhouni quipped—researchers have over-relied on animal data. "The problem is that it hasn't worked, and it's time we stopped dancing around the problem...We need to refocus and adapt new methodologies for use in humans to understand disease biology in humans."
Information on NIH grant support (funding) is taken verbatim from relevant publications. If you have difficulty with any links provided, you can do your own search through the NIH RePORTER site: https://reporter.nih.gov/, by copying and pasting the grant number into the Search field on the form.
Be aware that some grants include funds for more than experiments on non-human primates.
29 February 2024: USA imported over 1,400 wild-caught monkeys from Indonesia during 2023

Please join Action for Primates in urging the USA to stop importing long-tailed macaques from Indonesia. Our renewed call follows the disclosure that, during 2023, 1,402 long-tailed macaques captured from the wild in Indonesia were imported by the US research and testing industry. This represents an alarming increase of almost 40% since 2022, when the USA imported 870 wild-caught and 120 captive born long-tailed macaques.
In 2021, the government of Indonesia allowed the capture and export of wild long-tailed macaques to resume. This was despite widespread global concerns about the inherent inhumanity of trapping wild monkeys and increasing awareness of the vulnerability of the conservation status of this species. Since this resumption of wild trapping, the conservation status of the long-tailed macaque has been increased to Endangered with a decreasing population trend by the International Union for the Conservation of Nature (IUCN) Red List of Threatened Species (Hansen et al 2022).
Action for Primates has previously released harrowing video footage of the capture of wild long-tailed macaques in Indonesia which provides compelling evidence of the cruelty of the trappers and the suffering and distress caused to the monkeys; see video here and our previous Take Action here. This included brutal capture methods, the forced separation of nursing infants from their mothers and the beating and killing of unwanted individuals. Such inhumane treatment is a clear breach of international animal welfare guidelines (McCann et al 2007).
Long-tailed macaques are indigenous to Indonesia, part of the rich and diverse ecosystem, contributing to the country's unique biodiversity. The species, however, is not protected under Indonesian law and, in addition to the capture and export for the global research and testing industry, its wild populations face many other threats, including hunting for human consumption; capture as 'pets' or to be used in tourism and 'entertainment' activities, including the disturbing rise in baby macaque abuse videos filmed for broadcast on social media; and killing due to negative interactions with people.
Please take action for the long-tailed macaques in Indonesia by doing the following:
References:
8 February 2024: URGENT APPEAL – Stop construction of massive monkey farm in Mauritius

Action for Primates is deeply concerned over a proposal for another monkey farm to be established in Mauritius. The company, Hammerhead International Ltd, has submitted an application to the Ministry of the Environment with a proposal to build a facility that will eventually hold up to 12,000 long-tailed macaques. The company will initially be exporting around 4,000 wild-caught monkeys per year to laboratories where they will be subjected to experimentation and toxicity (poisoning) testing (like the monkey in the photo). If granted, this proposal would inflict even further suffering upon long-tailed macaques, including the capture of thousands of monkeys – 40-50 individuals every day, for over 300 days in the year, totalling up to 15,000 monkeys – an inhumane practice universally recognised as cruel because of the removal of the monkeys from their families, friends and and homes.
Urgent action is needed as we only have until the 24th February 2024 to submit objections to this application (see below).
Hammerhead International Ltd is a notorious company that made headlines in March 2023, when Mauritius authorities seized 446 long-tailed macaques – found to be kept in deplorable conditions – from an illegal farm it was operating. Its director, Shafeek Jhummun, was arrested and the Ministry of Agro-Industry and Food Security has brought a case against Hammerhead International for the ill-treatment of animals and for the illegal possession of macaques.

In recent years, Mauritius has exported many thousands of monkeys to laboratories in the USA, Canada, France, the UK, Spain, Germany and the Netherlands. Numbers have increased following the United States Fish and Wildlife Service investigation into the global trafficking of long-tailed macaques which led to restrictions on the export trade from Cambodia. The USA is now importing thousands of wild-caught monkeys from Mauritius. Long-tailed macaques are the primary non-human primate used in toxicity testing, which is the deliberate poisoning of animals to see whether and how much of a chemical or drug it takes to cause them serious harm or death. They are forcibly restrained, and a test substance given by injection, infusion, stomach tube or aerosol – in increasing amounts to measure the poisoning effects. The suffering caused to the macaques is immense and immeasurable.
Action for Primates has joined with other organisations, including People for the Ethical Treatment of Animals in the USA, One Voice, Abolición Vivisección and Cheshire Animal Rights Campaigns in Europe, and Monkey Massacre in Mauritius, in calling on the Government of Mauritius to refuse permission for this proposal. There is growing concern about the use of non-human primates in laboratories and a greater awareness of the genetic proximity of non-human primates to human beings together with their capacity to experience pain, suffering and distress similarly to people. We need to show the Mauritius government the strength of opposition to this cruel trade in monkeys' lives.
Please speak up for the monkeys in Mauritius:
2 February 2024: Long-tailed macaques subjected to brutal xenotransplant surgery in USA

Twenty-five long-tailed macaques lost their lives in a brutal experiment in which their kidneys were removed and replaced by those from mini-pigs (who also were killed) (Firl et al 2023). The work, which was done at the Massachusetts General Hospital in the USA, was almost entirely supported by eGenesis, a company which supplies xenotransplant organs and which supplied the mini-pigs used and killed in this research. The macaques were obtained from Charles River Laboratories, Alpha Genesis, Inc. and BC US LLC. Some of the macaques, supplied by BC US LLC, were stated to be wild-caught. BC US LLC is a breeding, holding and quarantine facility located in Florida. It is part of the Bioculture Group and imports wild-caught long-tailed macaques from its sibling company Bioculture (Mauritius) Ltd (BCM). In 2022, Bioculture (Mauritius) exported over 4,000 macaques – captured from the wild – to the USA.
In addition to the obvious vested interest of eGenesis in this work, three of the authors were being supported by or were employees of the company; two were stated to have equity interest in eGenesis. Three others had served as consultants to the company. Two of the researchers were supported by National Institutes of Health grant AI007529.
The procedures to which the macaques were subjected were reportedly conducted in accordance with the Guide for the Care and Use of Laboratory Animals...with the approval of the Massachusetts General Hospital Institutional Animal Care and Use Committee (Protocols 2017N000216 and 2017N000214)
.
The twenty-five long-tailed macaques were subjected to at least one major abdominal surgery to remove both their kidneys and implant the pig kidneys. Only 17 individuals lived past 60 days as eight were killed before this time, although no details were provided as to why this was done. The researchers acknowledged that this survival rate may not be representative of human xenotransplantation given certain incompatibilities of transplanting an organ designed for humans into NHPs [non-human primates] and challenges in NHP post-transplant care
.
Due to kidney function problems, four of the surviving macaques had had only one kidney removed initially. They were subjected to another major abdominal surgery about 20 days later to remove the other kidney. All the macaques were subjected to chemical immunosuppression so that they would not reject the pig kidneys. They were also subjected to multiple additional surgical procedures to remove pieces of the transplanted kidneys every two to four months, for evaluation. Survival periods for the macaques were stated to be from 64-648 days. It was not clear from the publication whether the macaques died or were killed (euphemistically referred to as euthanized
by the authors) because they were suffering beyond what was 'acceptable', including allowing the loss of 25% of body weight.
Eleven of the macaques developed hydronephrosis (stretched and swollen kidney due to build up of urine). The authors did not provide any details on the effects of the transplantation on the welfare and well-being of the macaques, other than to state that post-transplant care in non-human primates is 'challenging'. We know, however, that major abdominal surgery of this type (xenotransplantation) would result in considerable pain and suffering for an extended period, even if pain relief was provided. The authors provided no information on whether or what pain relief they may have provided the macaques.
The researchers acknowledged many serious limitations to their work, calling into question any applicability to humans:
limitations of the NHP model prevent assessment of more common and subjective features of hypercalcemia such as mood disorders, fatigue, or muscle weakness
in every transplant in this study, hypercalcemia and hypophosphatemia were observed...represents one of the main safety concerns
NHPs used in preclinical studies are healthy while potential human recipients of porcine xenografts will not be
the clinical implications of these findings on human recipients may be minimal
Research involving xenotransplantation – the transplantation of living cells, tissues or organs from one species to another – is some of the most gruesome work carried out on non-human animals. Such research raises many moral and welfare issues for the individuals who are genetically modified to be used as providers of organs, such as the mini-pigs killed in this experiment, and for those used in the research as recipients of the transplants. Xenotransplantation research involves the major trauma of the transplantation surgery and its inevitable consequences. In addition, the individuals suffer greatly from the immunosuppressive drugs they have to receive to prevent rejection of the transplanted organ, including internal haemorrhage, vomiting, diarrhoea and kidney or heart failure.
Action for Primates opposes xenotransplantation research and the 'creation' of genetically engineered non-human animals as living spare-parts 'warehouses' for people. Other options for addressing organ donor shortages should be considered, such as adopting an 'opt-out' approach to organ donations. In this case, unless explicitly expressed otherwise, the deceased is considered to have given tacit approval to be an organ donor. This would go a long way in increasing the availability of suitable organs for human-to-human transplants. The USA, however, seems intent on adopting xenotransplantation as a solution. Not only does the country operate an opt-in system for donor donation, in 2020, the US Food and Drug Administration (FDA) approved genetically engineered pigs for use in food and medical products.
What you can do to help:
Cited information:
Information on NIH grant support (funding) is taken verbatim from relevant publications. If you have difficulty with any links provided, you can do your own search through the NIH RePORTER site: https://reporter.nih.gov/, by copying and pasting the grant number into the Search field on the form.
Be aware that some grants include funds for more than experiments on non-human primates.
8 January 2024: Pregnant rhesus macaques fed marijuana and unborn infants killed in USA research

Rhesus macaques at Oregon Health & Science University, Oregon National Primate Research Center in the USA were made to consume marijuana edibles
before and during pregnancy and their unborn infants removed surgically and killed (Shorey-Kendrick et al 2023). The experiment was approved by the institution's animal use committee and was funded largely by public funds through grants from the National Institutes of Health: DA056493, HD000849, HD097116 and OD011092. Additional funding was provided through the March of Dimes Foundation and a Silver Family Innovation Award.
The macaques were used as 'models' of human chronic cannabis exposure
via the consumption of edible tetrahydrocannabinol (THC) consumption similar to that seen in humans. The researchers wanted to see if giving cannabis during pregnancy was harmful to foetal genetic material. Ten adult female rhesus macaques were used (five were used as 'controls'). They were housed indoors, but, contrary to accepted practice and recommendations (ARRIVE; Pomerantz et al 2022), there was no mention if they were singly housed and no other information on enrichment, etc, was provided. Because the monkeys were being fed a drug at a dose based on each individual, we have to assume that they were singly housed. The 'experimental' group was given tetrahydrocannabinol in cookies (edibles
) every day for about four months before being subjected to breeding to make them become pregnant, and throughout their pregnancies; the 'control' macaques were given a placebo. On gestational day 155, when the foetuses were almost full term (about 168 days), all the macaques were subjected to major survival surgery during which their foetuses were removed and killed. No details on the surgery were provided nor were there details on how the foetuses were killed.
The fate of the ten mothers after their use as 'foetus producers' was not mentioned. There was no stated consideration for the psychological trauma the mothers would have endured from the loss of their almost full-term infants, yet the mother-infant bond in non-human primates is similar to that in humans. We can only assume that these mothers were returned to the breeding 'colony' to be used again to produce more offspring for research. This is an exceptionally cruel fate for a species that is so like our own with respect to maternal bonding and attachment and developmental needs. Charities such as March of Dimes Foundation and Silver Family Foundation are committed to improving the health of mothers and babies and positive youth development. We cannot comprehend why they would choose to fund research that purposefully involved the infliction of such cruelty and suffering on non-human primate mothers and their unborn infants.
The researchers concluded that their results did not contribute substantially to our understanding of the long-term consequences of cannabis use on foetuses. Although they claimed that their limited results could be used to help counsel humans who use cannabis during pregnancy, they cited studies done on human beings, which demonstrated the effects of cannabis use on humans during pregnancy and beyond. The information necessary for counselling humans is already available. This study was done apparently without any regard for alternative, reliable and humane ways of getting information and was contrary to the Replacement criterion for the 3Rs.
The public are repeatedly told that non-human primates are used in research only when absolutely necessary and only when there are no other alternatives available. This shameful experiment, which resulted in substantial suffering for the monkeys and the death of their foetuses, demonstrates the meaningless nature of such assurances and a lack of commitment to stop using non-human primates. Not only is the information in macaques irrelevant to people given the fundamental differences between the two species, humane and ethical clinical studies have been and can continue to be done on human patients and volunteers in order to get data that are directly applicable to people.
What you can do to help:
Cited information:
Information on NIH grant support (funding) is taken verbatim from relevant publications. If you have difficulty with any links provided, you can do your own search through the NIH RePORTER site: https://reporter.nih.gov/, by copying and pasting the grant number into the Search field on the form.
Be aware that some grants include funds for more than experiments on non-human primates.
![]()
![]()
![]()
![]()
![]()
Contact us via E-mail
Copyright © 2020-2026 Action for Primates. All rights reserved.